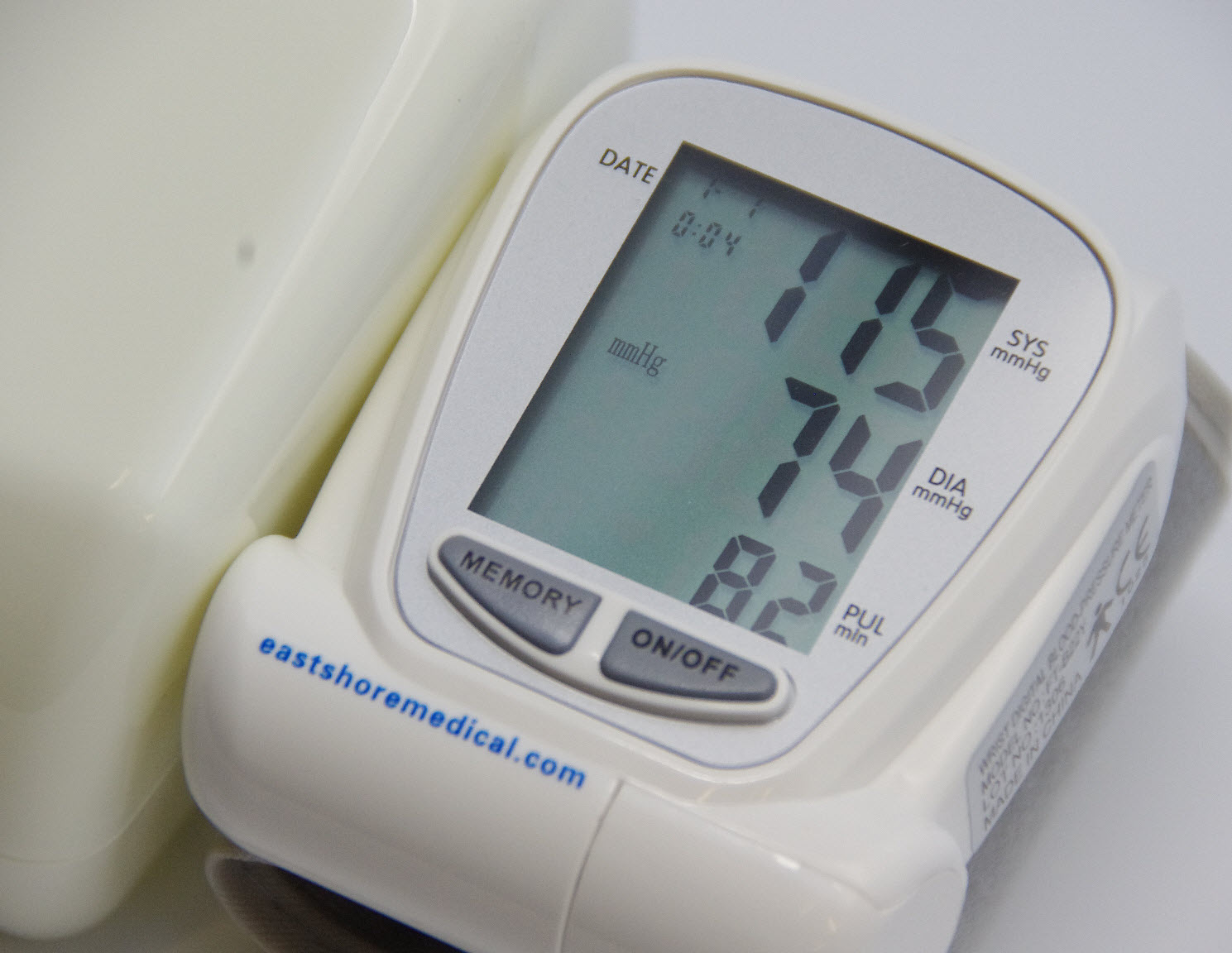
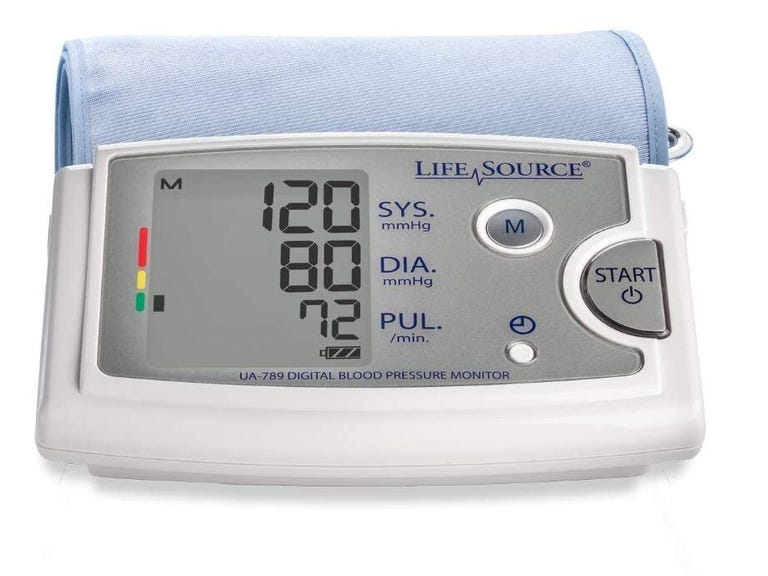
US FDA grants 510(k) for CardieX's arterial health monitor
$ 20.99
-
By A Mystery Man Writer
-
-
5(155)

Product Description
The US Food and Drug Administration (FDA) has granted 510(k) clearance for CardieX’s CONNEQT Pulse, a new arterial health monitor.

CardieX - Home

Most recent hypertension related innovations

Most recent hypertension related innovations

Sameer Molvi on LinkedIn: CardieX Selected for Medtech Innovator's 2023 Accelerator Cohort

blood pressure MobiHealthNews

formdrs_01.jpg

Quantra Hemostasis System by HemoSonics Gets FDA 510(k) Clearance - Xtalks

Arterial health monitor CONNEQT Pulse gains FDA 510(k) clearance
Bluetooth Medical Devices Cleared by FDA in 2023

Hasini Devarasetti, Author at Medical Device Network